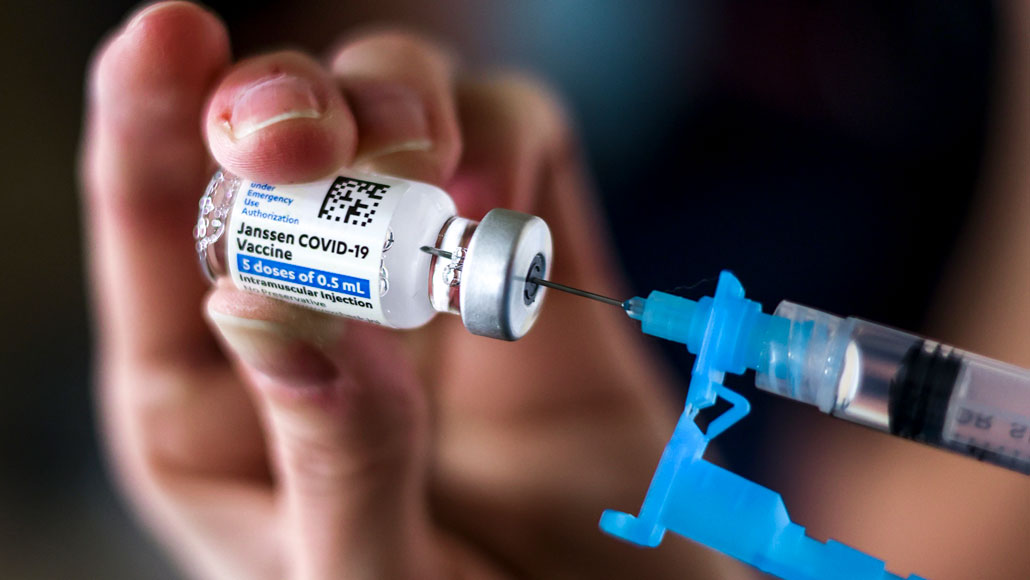
Infusions of Johnson and Johnson's Covid immunization went to an unexpected stop the nation over on Tuesday after government wellbeing offices required a respite in the antibody's utilization as they look at an uncommon blood-thickening issue that arose in six beneficiaries.
Every one of the six were ladies between the ages of 18 and 48, and all built up the sickness inside one to three weeks of inoculation. One lady in Virginia passed on, and a second lady in Nebraska has been hospitalized in basic condition.
In excess of 7,000,000 individuals in the United States have gotten Johnson and Johnson shots up until now, and another 10 million portions have been delivered out to the states, as per information from the Centers for Disease Control and Prevention.

"We are suggesting a delay in the utilization of this immunization out of a wealth of alert," Dr. Peter Marks, the overseer of the Food and Drug Administration's Center for Biologics Evaluation and Research, and Dr. Anne Schuchat, the central agent head of the C.D.C., said in a joint explanation. "At the present time, these antagonistic occasions give off an impression of being incredibly uncommon."
While they outlined the move as a suggestion to wellbeing specialists, the effect was prompt. By Tuesday evening, each express, the District of Columbia and Puerto Rico had reported a respite in Johnson and Johnson immunization infusions.
The equivalent went for the U.S. military, governmentally run inoculation locales, and CVS and Walgreens, two drug store monsters that partake in the bureaucratic program, authorities said. Ceremony Aid, Walmart and Publix additionally reported that they had stopped Johnson and Johnson infusions.
Past American shores, Johnson and Johnson said it would defer the rollout of its immunization in Europe, where a few nations were ready to begin managing it this week. South Africa, crushed by a more infectious variation of the infection that arose there, additionally suspended utilization of the antibody. Australia reported it would not buy any portions.

The Biden organization attempted to depict itself too ready for the misfortune. President Biden said he would meet his objective to have enough dosages to inoculate each American grown-up who needed it before the following month's over.
The response provoked an exceptional discussion among general wellbeing specialists about whether guarding against a particularly uncommon problem merited the expense. Scores of immunization arrangements were dropped for this present week, and some general wellbeing authorities expected that by filling antibody aversion and trick scholars, the respite could incite less Americans to get inoculated — and open them to undeniably more danger.
Others said the F.D.A. also, the C.D.C. just had no way out.
"It's amazingly difficult, yet to disregard it would have been more terrible," said Rachael Piltch-Loeb, a specialist in wellbeing hazard correspondence at the N.Y.U. School of Global Public Health. In the event that the public speculated that the public authority was covering genuine expected results, she said, undeniably more individuals may rule against immunization.
Dr. Janet Woodcock, the acting chief of the F.D.A., said at a news gathering that the interruption may last just "only days," despite the fact that she said that relied upon "what we realize in the following not many days." Dr. Schuchat said the respite was established somewhat to "set up the medical services framework to perceive and treat patients properly."
Researchers with the F.D.A. furthermore, the C.D.C. will together look at potential connections between the immunization and the problem and decide if the F.D.A. should keep on permitting crisis utilization of the immunization for all grown-ups or alter the approval, potentially by restricting the antibody to certain populace gatherings. A crisis meeting of the C.D.C's. outside antibody warning council has been booked for Wednesday.
In spite of Mr. Biden's confirmation, the delay will convolute the country's immunization endeavors when numerous states are defying a flood in new cases and looking to address antibody reluctance. Controllers in Europe and somewhere else are worried about a comparative issue with another Covid antibody, created by AstraZeneca and Oxford University analysts.
At the news meeting, Dr. Imprints drew a connection between the two immunizations, saying the coagulating cases were "extremely, comparative." The antibodies depend on comparable innovation, yet AstraZeneca's has not yet been approved for crisis use in the United States.
By far most of the country's antibody stock comes from two different makers, Pfizer-BioNTech and Moderna. Those two antibodies utilize an unexpected innovation in comparison to Johnson and Johnson's and AstraZeneca's. In excess of 180 million portions of Pfizer and Moderna have been directed, as per the C.D.C's. most recent insights, and government authorities pushed on Tuesday that they had seen no proof of the blood coagulations that prompted the respite of the Johnson and Johnson antibody — or of some other critical security concern.
The choice is a new hit to Johnson and Johnson. Before the end of last month, the organization found that laborers at a Baltimore plant had coincidentally defiled a group of its immunization, constraining the firm to toss out great many dosages. With government affirmation of that processing plant in question, Johnson and Johnson's shipments dropped to one-fourth or less of what had been normal, an extreme disillusionment to White House and state authorities.
Jeffrey D. Zients, the White House facilitator of the pandemic reaction, said that the government was all the while dispatching out 28 million Pfizer and Moderna portions this week and that around 3,000,000 shots were being managed every day.
Yet, the White House had been expecting a far better appearing. At a certain point this spring, authorities were anticipating week after week shipments of in excess of 4,000,000 dosages of Johnson and Johnson antibody starting this month.
Since Johnson and Johnson is one portion and effectively put away, it was bound for particular antibody outreach programs. In Colorado and California on Tuesday, portable immunization centers in country regions were dropped. In Chicago, immunization occasions for café representatives and avionics laborers were deferred uncertainly. At schools in Ohio, New York and different states, where the one-portion immunization offered an opportunity to rapidly vaccinate understudies before they left grounds for summer, arrangements were canceled as a group.
With just two immunizations rather than three, government authorities hope to have enough portions to cover all things considered 230 million grown-ups before the finish of May, approximately 30 million short of the complete grown-up populace. However, regardless of public missions to persuade them, a specific portion of grown-ups are required to decline shots, so that supply may cover all the interest.
Government specialists are worried that specialists may not be prepared to spot or treat the uncommon issue if beneficiaries of the antibody create indications. Dr. Imprints said that a standard treatment for blood clusters — utilization of an anticoagulant drug called heparin — "can really cause enormous damage, or the result can be lethal."
The C.D.C. what's more, the F.D.A. suggested that individuals who have gotten the Johnson and Johnson immunization inside the previous month contact their PCPs in the event that they experience extreme migraines, stomach torment, leg agony or windedness. Authorities said the most well-known side effect of the problem was a persevering, moderate to serious cerebral pain that starts six days or later after the shot.
Dr. Schuchat, the C.D.C. official, said the danger of hazardous blood clumps was "extremely low" for individuals who got Johnson and Johnson's antibody over a month prior.
In a proclamation, Johnson and Johnson said it upheld "open correspondence" with medical care experts and general society and had been working intimately with clinical specialists and wellbeing specialists, remembering for Europe. "We have settled on the choice to proactively defer the rollout of our immunization" there, the firm said.
The organization likewise said that it was stopping immunizations in its clinical preliminaries. It is as of now testing the adequacy of two dosages, instead of the single portion that was approved in February.
In the United States alone, 300,000 to 600,000 individuals a year create blood clumps, as per C.D.C. information. However, the six cases that prompted the respite included an uncommon blend of manifestations. The coagulations happened in the mind, in a condition called known as cerebral venous sinus apoplexy. The coagulations were joined by low degrees of platelets, a part of blood that helps structure clusters that regularly help recuperate wounds.
The entirety of the ladies built up the disease inside around one to three weeks of inoculation, with a middle season of nine days. The hospitalized Nebraska lady is in her late 40s and created blood clumps fourteen days after her shot, state wellbeing authorities said.
The Virginia lady, 45, created side effects six days after she was vaccinated toward the beginning of March and passed on six days from that point forward, wellbeing authorities said. They said that the public consideration is now creating a spate of new reports of potential cases to government specialists.
Government authorities said there was expansive arrangement in the senior positions of the organization about the need to stop utilization of the antibody while they explore whether the blood coagulations are connected to a safe framework reaction created by the immunization.
Dr. Imprints said the central government had not arranged the immunization's suspension since wellbeing suppliers may conclude that for a specific patient, the advantages of a shot exceed the dangers. "We're not going to prevent that supplier from overseeing the antibody since it very well may be correct," he said.
The worries about Johnson and Johnson's immunization reflect those about AstraZeneca's. European controllers started researching that antibody a month ago after certain beneficiaries created blood clumps and low platelet checks like those of the Johnson and Johnson beneficiaries.
Out of 34 million individuals who got AstraZeneca's shots in Britain, the European Union and three different nations, 222 experienced blood clusters connected to a low degree of platelets. A greater part of these cases included ladies under 60.
On April 7, the European Medicines Agency, the principle administrative office, presumed that the turmoil was an exceptionally uncommon result. The European controllers contended that the advantage of the AstraZeneca antibody inconceivably exceeded that little danger. Nations in Europe and somewhere else have kept on giving the antibody to more established individuals, who are more in danger from the Covid, while limiting it in more youthful individuals.
Both Johnson and Johnson and AstraZeneca use adenoviruses to convey DNA into human cells to start the way toward producing invulnerability to the Covid. It isn't yet known whether that common innovation causes coagulating in uncommon cases.
Specialists have guessed that DNA stole by the adenoviruses may set away the safe response in certain individuals. The condition might be exceptionally uncommon on the grounds that individuals must have some attribute — as yet unclear — that inclines them to this breakdown.
The principal public indication of worry about Johnson and Johnson's antibody went ahead April 9, when the European Medicines Agency declared that it was examining reports of four instances of blood clusters in individuals who got the immunization in the United States. One case happened in the clinical preliminary that occurred before the immunization was approved. Three happened in the immunization rollout. One of them was deadly, the office said. The controllers said the bunch of cases required examination, yet that it was not satisfactory whether the antibody was to be faulted.



Comments
Post a Comment